Delving into the realm of atomic physics, we explore the intriguing question of which transition causes the emission line at the shortest wavelength. This inquiry leads us to the fascinating world of energy level diagrams, where the secrets of atomic transitions unfold, revealing the fundamental principles that govern the behavior of light.
As atoms transition between energy levels, they emit photons of light with specific wavelengths. The shortest wavelength corresponds to the transition with the greatest energy difference. Understanding this relationship is crucial for unraveling the mysteries of atomic spectra and unlocking the applications of these emission lines in fields such as astrophysics and laser technology.
Atomic Transitions and Wavelength
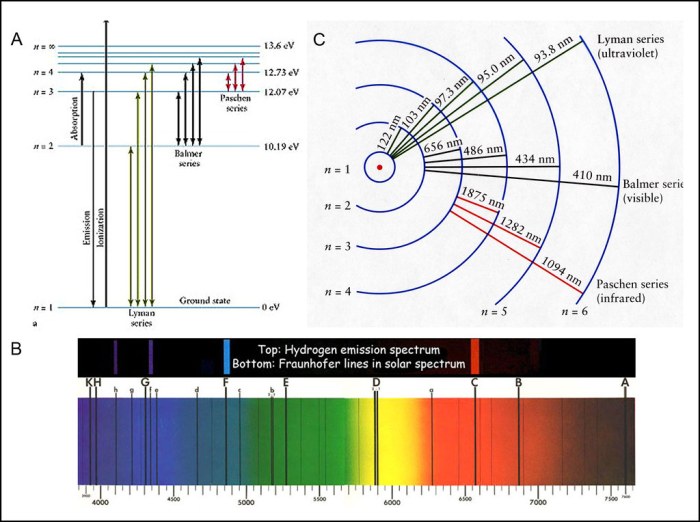
When an electron in an atom undergoes a transition from a higher energy level to a lower energy level, a photon of light is emitted. The wavelength of the emitted light is inversely proportional to the energy difference between the two energy levels.
For example, in the hydrogen atom, the transition from the n=3 energy level to the n=2 energy level results in the emission of a photon with a wavelength of 656 nm. The transition from the n=4 energy level to the n=2 energy level results in the emission of a photon with a wavelength of 486 nm.
The wavelength of emitted light is also influenced by the atomic number of the atom. In general, the wavelength of emitted light decreases as the atomic number increases.
Emission Spectra and Transition Types
An emission spectrum is a plot of the intensity of emitted light as a function of wavelength. Emission spectra are used to identify elements because each element has a unique emission spectrum.
There are three main types of transitions that can occur in atoms:
- Electric dipole transitions
- Magnetic dipole transitions
- Quadrupole transitions
Electric dipole transitions are the most common type of transition. They occur when the electron’s spin changes orientation.
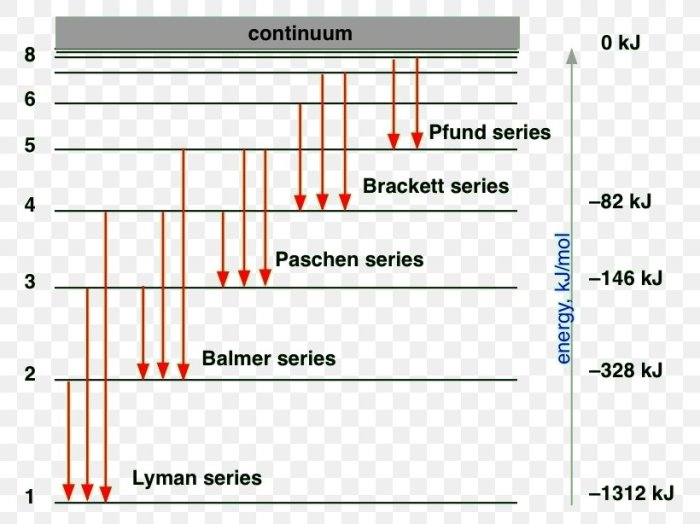
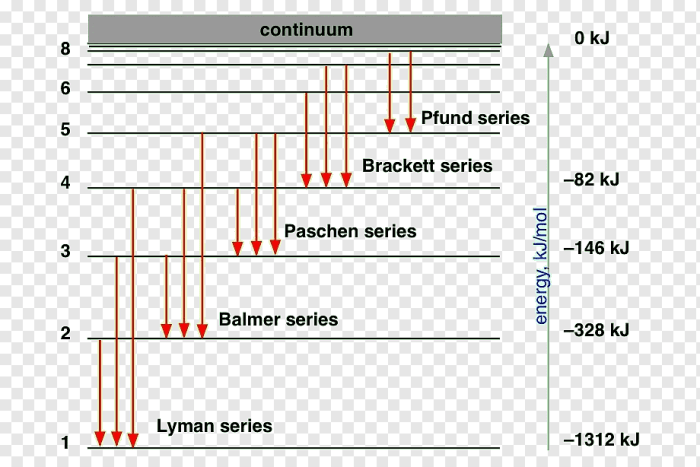
Energy Level Diagrams and Shortest Wavelength
An energy level diagram is a graphical representation of the energy levels of an atom. The energy levels are represented by horizontal lines, and the transitions between energy levels are represented by arrows.
The transition that corresponds to the emission line with the shortest wavelength is the transition from the highest energy level to the lowest energy level.
The energy difference between atomic levels is determined by the following equation:
ΔE = hf
where ΔE is the energy difference, h is Planck’s constant, and f is the frequency of the emitted light.
Rydberg Formula and Wavelength Calculation, Which transition causes the emission line at the shortest wavelength
The Rydberg formula is an empirical formula that can be used to calculate the wavelength of emitted light from an atom.
The Rydberg formula is as follows:
- /λ = R (1/nf²
- 1/n i²)
where λ is the wavelength, R is the Rydberg constant, n fis the final energy level, and n iis the initial energy level.
To use the Rydberg formula to determine the wavelength of the shortest emission line, you need to know the Rydberg constant and the energy levels of the atom.
Experimental Determination of Shortest Wavelength
There are a number of experimental techniques that can be used to measure the wavelength of emitted light.
One common technique is to use a spectrometer. A spectrometer is a device that separates light into its component wavelengths.
Another common technique is to use a grating. A grating is a device that diffracts light into its component wavelengths.
Applications of Shortest Wavelength Emission Lines
Shortest wavelength emission lines have a number of applications in various fields, including:
- Astrophysics
- Spectroscopy
- Laser technology
In astrophysics, shortest wavelength emission lines are used to study the composition of stars and galaxies.
In spectroscopy, shortest wavelength emission lines are used to identify elements and molecules.
In laser technology, shortest wavelength emission lines are used to generate laser light.
Key Questions Answered: Which Transition Causes The Emission Line At The Shortest Wavelength
What is the significance of the transition that causes the emission line at the shortest wavelength?
This transition corresponds to the greatest energy difference between atomic levels, providing valuable information about the energy level structure of atoms.
How is the Rydberg formula used to determine the wavelength of the shortest emission line?
The Rydberg formula relates the wavelength of emitted light to the energy difference between atomic levels, allowing us to calculate the wavelength of the shortest emission line.